|
Home
About
Us
Resources
Bookstore
Education
Support
SII
Research
Contact
Us
|
Return
to Previous Lecture Series page
Energy and Everyday
Experience (Part 1 of “Key Concepts of Physics” Series)
Todd Duncan
Science Integration Institute
Last modified 6/15/02
Introduction
The concept of energy is an ideal topic with which to begin this
series of essays because it plays a role in literally everything
we do in ordinary daily life. We’re all certainly familiar
with the term in everyday conversation. The so-called energy shortage
in California has been in the news during the past couple of years,
last year’s droughts in the Northwest left concerns about the
supply of energy from hydroelectric dams, and we are generally encouraged
to conserve energy in order to reduce dependence on foreign fossil
fuel and to minimize our impact on the environment. We even come
face to face with precise quantitative values of energy when we
pay our power bill each month. But how well do we really understand
what a “kilowatt hour” is or how that number on our power
bill is connected to specific resources we use up or other impacts
we have on our environment? Despite our passing familiarity with
energy, the deeper principles it expresses about how the universe
basically works, and how these principles express themselves in
our attempts to rearrange parts of the world to suit our preferences,
are generally not widely understood.
So this is why I say energy is a concept that fits perfectly within
the theme of science integration and of this series. It is a familiar
and integral part of our everyday experience with the world (everything
we do involves a transfer of energy from one form to another), yet
it is also a very subtle and mysterious concept which leads us into
some of the deepest and most profound questions about the universe
and our relationship to it. In addition, it is a key idea to grasp
for anyone who wants to read and understand any popular subject
in physics, since the notion of energy is one of the conceptual
building blocks for all of modern physics.
My aim in this paper is to provide an introduction to energy that
lets you see how the concept developed from our direct experience
with the world, how it connects to your own everyday experiences,
and how it can provide an organizing and unifying principle to help
you make sense of (and experience the deep mystery of) the connections
and patterns you observe in the world of which you are a part.
Development of the concept from our experience with the world
Although the modern, formalized concept of energy and the related
laws of physics can seem quite abstract and unfamiliar, this abstract
concept emerged as a way to organize and refine the description
of our very common and familiar experiences with the world. I’d
like to begin by tracing a little of this conceptual development
to provide a direct link between experiences everyone can relate
to, and the generalized concept of energy as it is used in physics.
So first just a few immediate observations to get you thinking about
some everyday experiences: You get tired when you climb a long flight
of stairs. You get hungry if you exercise for a long time without
eating. A lamp won’t emit light if it isn’t plugged into
a power outlet. Your car stops moving if it runs out of gas. You
can be cold lying on a beach under cloud cover, and turn toasty
warm in a matter of minutes when the sun comes out. You speed up
as you coast downhill on a bicycle, and you slow down again if you
try to coast back up the next hill.
All these experiences have the common feature that a particular
change you want to make to the state of the world is accompanied
by other, corresponding changes in the state of things: You can
coast from the bottom of the hill to a point parkway to the top,
but your speed will necessarily be slower at the new position. You
can move from the bottom of the stairs to the top, but not without
feeling a little more tired. You can make your car move, but not
without using up gasoline. You can make yourself feel warmer while
lying on the beach, but not without sunlight streaming down upon
you. What I'd like to explore now is whether we can extract some
kind of general principle from these observations. Is there a unifying
principle that we could carry through our description of all the
various possible arrangements, that summarizes and captures the
different constraints on how things can be arranged? How can we
express these kinds of limitations or constraints in more general
terms, in a language that lets us think about a wide variety of
such situations with relatively few basic concepts?
The examples we’ve already discussed point to a very vague
idea of this principle, something we know quite generally from our
experience with the world: some things are possible for us to do,
some are impossible, and there are specific constraints and limitations
on how we must arrange things in order to make the possible things
happen. For example, if I want to go visit my friend in Seattle,
this is possible, but not without gasoline in my car. In order to
allow the process that moves me from here to Seattle, molecules
in the gasoline must break apart and combine with oxygen to form
other molecules. But why can’t I make my car move without gasoline?
What basic property of the world imposes that limitation? In order
to make anything happen, we have a vague sense that there must be
something to supply the “capacity” to make that thing
happen. This is expressed in the commonsense wisdom that there is
no “free lunch” or “you can’t get something
for nothing.” This is also the hard lesson learned from hundreds
and even thousands of years of efforts by inventors to develop “perpetual
motion machines” that would cause some desirable change to
the state of the world (lifting a weight or turning a wheel or running
an engine or any of a variety of actions that might be useful to
us) without any cost or change to the rest of the world. The lesson
is that in order to change the state of something in a particular
way that you’d like to, you need some sort of “capacity”
to cause that change, and this capacity must be taken away from
something else in the world and given to the thing you want
to change. This is a refinement of our earlier observation that
if you want to change one thing, you can never do so in such a way
that you change nothing else at all about the state of the world.
Let’s try to sharpen this idea a little further by zeroing
in on the notion that there is “something” that gets passed
along or transferred as the “ability to make interesting things
happen” moves from one part of the world to another. A specific
example involving a long series of processes will be helpful here
in seeing that there really is some kind of specific capacity to
make things happen that moves along the chain. So let’s imagine
a chain of events that starts inside the sun with the combining
of hydrogen atoms into helium, where these atoms give up something
that is passed along to the light that streams toward the earth.
Then the light is absorbed by some plants, transferring this something
from the light into the plants to enable them to grow. Then we eat
the plants, and gain the power to make interesting things happen
like typing a few words in the computer or jumping off the floor
a little bit or really anything you could imagine doing.
So far this idea is just a rough, qualitative statement. In order
to understand that this “something” is what we now call
energy, and in order to see the full power of the concept, we need
to move beyond qualitative statements and learn how to assign specific
numerical values to this thing that gets passed from one part of
the world to another and that represents the capacity to make interesting
things happen. Once we know how to calculate this specific quantity
in different situations, we’ll see that we can make a much
more sweeping statement than our previous observation that to change
one thing about the world, there must be a corresponding change
in something else. We’ll find in fact that the amount of one
thing you have to give up to get a certain amount of the other thing
is always the same. In order to describe this, I need to
formalize this capacity to make interesting things happen into a
precisely measurable quantity, a quantity we call energy.
Formalizing the concept: the law of conservation of energy (first
law of thermodynamics)
Let’s see if we can formalize these rough observations into
a more precise general principle. To any state of some part of the
world ( “system”) that we observe, we can assign a quantity
called energy which is calculated in terms of the properties
that characterize the state of the system. There are very specific
rules for calculating the amount of energy to assign to specific
properties of a system. For example, if the system is very simple
and consists only of a single particle of mass m moving at
speed v, then m and v are the only two properties
characterizing the system. The energy we should assign in this case
has been found to be given by multiplying the value of the mass
times the square of the speed and dividing by two, which is the
probably familiar formula for energy stored in the form of motion,
or kinetic energy, 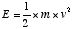 . Another
example is a system that stores energy in the form of gravitational
potential energy, given by . Another
example is a system that stores energy in the form of gravitational
potential energy, given by 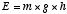 , where g
is the acceleration of gravity and h is the height of the
object above a reference point such as the ground. Another familiar
equation expresses the energy stored in the fact that an object
has mass, , where g
is the acceleration of gravity and h is the height of the
object above a reference point such as the ground. Another familiar
equation expresses the energy stored in the fact that an object
has mass, 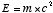 , where c, the speed of
light, has the value 300,000 km/s. So a very small amount of mass
stores a tremendous amount of energy, a fact which is made shockingly
apparent when a nuclear explosion converts a small amount of mass
into a tremendous amount of energy in other forms. One other specific
formula is for energy in the form of heat, E= (Heat Capacity)
x Temperature. This relationship was a very important discovery
in the history of science because it showed that energy in the form
of heat can be interchanged with other more mechanical forms of
energy. Thus it opened the way for understanding that energy is
something that is never lost. , where c, the speed of
light, has the value 300,000 km/s. So a very small amount of mass
stores a tremendous amount of energy, a fact which is made shockingly
apparent when a nuclear explosion converts a small amount of mass
into a tremendous amount of energy in other forms. One other specific
formula is for energy in the form of heat, E= (Heat Capacity)
x Temperature. This relationship was a very important discovery
in the history of science because it showed that energy in the form
of heat can be interchanged with other more mechanical forms of
energy. Thus it opened the way for understanding that energy is
something that is never lost.
There are many other forms of energy: light carries energy, chemical
bonds store energy, energy is stored in magnetic fields, etc. The
central point is that the energy is a specific number you can calculate
associated with each configuration of a system, and there is a well-defined
procedure for computing the energy in terms of the parameters describing
the system. This concept of an energy that we can compute is just
a quantitative refinement of the general concept we discussed earlier,
of a “something” that gets passed along from one part
of the world to another and represents the capacity to make interesting
things happen.
This quantitative measure of energy allows us to formulate one of
the most general and deep of all the laws of physics: the law of
conservation of energy (also known as the first law of thermodynamics).
It’s an articulation and refinement of the general experience
with nature I’ve been describing -- our inability to make certain
things happen without specific corresponding changes in the configuration
of some part of the surroundings.
The law says that the total amount of the quantity called energy
in the universe is conserved. This means you can take energy
from one place and move it somewhere else, or change it from one
form into another (e.g. from energy in sunlight to energy in the
form of hot water). But if you add everything up, making sure nothing
slipped away unnoticed, the total amount of energy stays the same.
One useful way to summarize this idea is to say that the energy
lost in one place always equals the energy gained in another place.
It’s worth pausing to consider how amazing and useful this
law is in understanding a wide variety of things that happen in
the world. Energy can be stored in all kinds of different forms
as we’ve just discussed For each form in which the energy might
be stored, the setup is totally different. In one case we might
be talking about a cup of hot water, in another we might be talking
about a tank of gasoline, in another a book about to fall from the
edge of a table, in another a baseball flying through the air. And
for each of these cases, the way we use the properties of the system
to calculate how much energy it has is totally different (one case
uses height and mass, another speed and mass, another heat capacity
and temperature, yet another the strength of an electric or magnetic
field, etc.). But once we've used these formulas to calculate this
mysterious quantity we call energy for one setup, then we can forget
about the specific details of that setup. This single quantity,
the energy, is all we need to know to determine if it is possible
in principle to make our car run, heat the water to a certain temperature,
etc. You tell me what you want to be able to do and how to calculate
energy for that configuration, and I’ll tell you if it’s
possible. For example, you might tell me how much energy is needed
to heat your house, and I can tell you if the water falling at Bonneville
Dam is enough to do it. Imagine how difficult that would be if I
actually had to follow all the details of how the falling water
sends current through the wires, runs your heater, affects the air
molecules, etc. Or if I had to follow all the details of all the
forces in a car, rather than just knowing how much energy is in
a tank of gas.
Next I want to give a few examples to show exactly how this idea
works in practice, but to do that I first need to introduce some
units for measuring energy.
Quantifying our understanding of energy: units for measurement
One great benefit made available to us by the law of conservation
of energy is that we may pick any form we like as a standard of
reference for measuring the amount of energy stored in any other
form. For example, we might pick up a handy thermos full of water,
stick a thermometer in it, and define our basic unit of energy as
that amount necessary to raise the temperature of the water by 10°C.
Then if you give me some energy in any form whatsoever, and ask
me how much that is, all I have to do to express it in terms of
my newly-invented unit is extract the energy from the form it’s
in now, put it to work heating the water, and see how much the temperature
goes up. For example, I might have a weight that has been raised
to a certain height. I know that there is energy stored in this
configuration because I can use it to turn a generator, or more
obviously, because it hurts if it falls and hits me on the head!
If I want to measure the amount of energy in terms of my newly invented
unit, I could tie the weight to a string, hook up the string so
it turns a paddlewheel that stirs the water as it turns, and then
let the weight fall in such a way that its gravitational potential
energy is converted into the turning of the paddle wheel and thereby
into heating the water. Then I just read the thermometer to see
how much energy it is (e.g. if it heats the water by 20 °C then
it’s 2 of my units of energy). Of course, I have to be careful
that I truly harness all of the energy, and don’t lose any
in friction in the other parts of the system that doesn’t go
into heating the water. There are many complications in getting
an accurate measurement of the amount of energy in practice. But
you get the idea, and hopefully you see that this can work in principle
with energy in any form: light has energy and will heat the water,
sound has energy, etc. In fact this is the fundamental way to determine
the energy associated with any particular phenomenon we might observe.
This new thermos-based unit of energy is not terribly convenient
as a standard of measurement because if someone wanted to duplicate
my system of measure, they’d have to track down a thermos of
water and be sure it was the same as the one I used. I used it as
an example merely to show you that it really is possible to use
anything as your standard of measure, and the units we are
familiar with are just the result of everyone agreeing on the equivalent
of what kind of “thermos” to use. So for example a widely
used unit of energy is the calorie, defined as the amount
of energy required to raise one gram of water (at 14.5°C) by
1°C. The only essential difference really between this and our
made up “thermos” unit of energy is that the units involved
are more standard and widely known, so they are more easily communicated
to and duplicated by others.
A few other units need to be introduced in order to talk in more
detail about how energy applies in daily life. Keep in mind that
any of these units can be referred back to something as concrete
as heating water in a thermos bottle.
• joule -- Another unit of energy, equal to the amount
of work done in exerting a force of 1 Newton (1 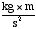 )
through a distance of 1 meter. 1 calorie is 4.2 joules, so 1 food
Calorie is equivalent to 4,200 joules. )
through a distance of 1 meter. 1 calorie is 4.2 joules, so 1 food
Calorie is equivalent to 4,200 joules.
• watt -- A unit of power, which measures the
rate at which energy is transferred from one form to another.
In general, power has units of energy divided by time; so just as
energy and time can be measured in many different units, so can
power. The watt is defined as 1 Joule of energy transferred
each second.
• kilowatt hour (kWhr) -- Yet another unit of energy,
which you have probably seen on your power bill. The odd unit (“1000
Watts times 1 hour”) arises because we measure the rate of
energy flow from the power company in the convenient unit of a kilowatt
(1000 watts or 1000 Joules transferred per second) and multiply
it by the number of hours during which we draw power. So a kilowatt
hour is also (1000 Joules/second) x 1 hour = 3.6 million Joules.
This is an odd but perfectly correct unit of energy. It’s analogous
to measuring distance in units of something like “miles per
hour times seconds.” Normally you would multiply miles per
hour by the number of hours traveled, to get miles traveled. But
you could also multiply by another unit of time such as seconds,
and it’s still a valid distance.
So now with these units in hand, we can trace in more detail the
kinds of transformation processes we discussed before. When you
put a teakettle on the stove and heat up the water, that takes a
certain amount of energy (which depends on the heat capacity of
the water and kettle, and on how much you want to raise the temperature).
Where does that energy come from? The burner on the stove must lose
that amount of energy, according to our law of conservation of energy.
And the burner got it from the electricity that came through the
power lines, which may have come originally from the gravitational
potential energy of falling water over a dam or from the chemical
energy stored in the coal. The point is that now we can calculate
exact amounts and actually know what had to be given up in order
to make the process work. All these forms of energy are interchangeable
at least in principle. Knowing how many Calories you have eaten
(say 2000 Calories) tells you how much water could be heated by
the energy stored in that food.
So as a specific example to illustrate the usefulness of the connections
between different forms of energy, we might ask about how much food
is needed in order to climb a small mountain. In order for a person
with a mass of 50 kg to get to the top of a mountain 1000 m tall,
we need to take away from the food an amount of energy
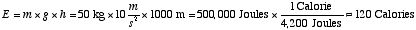 . .
Of course this is a bare minimum; we expect to need more than 120
Calories to climb the mountain because our bodies are far from perfectly
efficient in transforming food energy into gravitational potential
energy. Much of the energy goes into heat as part of the process
of moving us up the mountain. But in any case you can get the idea
that there is a direct relationship between the amount of food available
and the height to which we are able to climb -- quantities that
at first glance have nothing at all to do with one another.
The next example will also serve to address a question that may
have occurred to some of you: Why do people worry so much about
“conserving energy” if it is a fundamental law of nature
that energy can never be created or destroyed and so is always
conserved? The reason is that there are really two tests of the
situation to think about in deciding whether a given process can
occur:
1) First test: Is the energy necessary for that process available
in some form? Without this, there is no possible way to get any
further. Any configuration has a certain amount of energy associated
with it, and if there is no possible source of that energy, there
is no way for that process to occur.
2) Second test, a refinement of the first: Is the energy in a form
(or can it be converted to a form) that can be used by the process
or structure of interest? For example, we can calculate how much
energy we need, in food, to get through a day or to climb a mountain.
It turns out that a daily food requirement of 2000 Calories is about
the amount of gravitational potential energy stored in a 100 kg
weight held at a height of 10 km above the surface of the earth.
(You can use the units I’ve summarized in the next section
to check this if you’d like). So in principle, if you are hungry,
a 100 kg weight falling on you from a height of 10 km should be
able to appease your hunger and provide you with your daily supply
of energy. Of course, I don’t recommend trying this! Your body
has no mechanism for converting the energy in the falling weight
into forms that drive the chemical processes that keep you alive.
However, you are still better off having the gravitational potential
energy available than if you had no source of energy at all. You
could for example use the falling weight to turn a generator that
powers a light which allows plants to grow that you could then eat
as food; effectively packaging the gravitational potential energy
in the form of chemical bonds that your body is able to use to extract
the energy it needs for its life processes.
So when we talk about conserving energy, what we really mean is
conserving energy stored in forms that are useful to us for the
things we want to make happen. Gasoline, for example, is a much
more useful form of energy than the heat stored in the random motions
of molecules after the gasoline has been used to drive our car around.
The energy is all still there, it just isn’t in forms that
are as useful to us. We’ll discuss this idea much further in
the second essay.
Summary of useful units and relationships
I’ve discussed previously some units for measuring energy and
related quantities, along with useful relationships to keep in mind
among some of these quantities. Below is a summary of these for
handy reference.
• calorie -- The amount of energy required to raise
the temperature of 1 gram of water by 1 °C (starting at a standard
reference temperature of 14.5°C). (Note that the commonly used
food Calorie (capital “C”) is equal to a kilocalorie
or 1000 calories. For reference, a typical daily intake is 2000-3000
Calories.)
• joule -- Another unit of energy, equal to the amount
of work done in exerting a force of 1 Newton (1 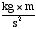 )
through a distance of 1 meter. 1 calorie is 4.2 joules, so 1 food
Calorie is equivalent to 4,200 joules. )
through a distance of 1 meter. 1 calorie is 4.2 joules, so 1 food
Calorie is equivalent to 4,200 joules.
• watt -- A unit of power, which measures the
rate at which energy is transferred from one form to another.
In general, power has units of energy divided by time; so just as
energy and time can be measured in many different units, so can
power. The watt is defined as 1 joule of energy transferred
each second.
• kilowatt hour (kWhr) -- Yet another unit of energy,
which you have probably seen on your power bill. The odd unit (“1000
watts times 1 hour”) arises because we measure the rate of
energy flow from the power company in the convenient unit of a kilowatt
(1000 watts or 1000 joules transferred per second) and multiply
it by the number of hours during which we draw power. So a kilowatt
hour is also (1000 joules/second) x 1 hour = 3.6 million joules.
• solar energy -- The flux of power pouring onto the
Earth in the form of sunlight (ignoring reflection and averaging
over the Earth’s surface) is about 342 Watts/m2.
Since most of the energy available to us on Earth derives ultimately
from this influx of sunlight, keeping this number in mind is handy
as a point of comparison for different energy sources and the energy
requirements of various appliances and activities we like to make
possible. (Compare to some typical power requirements for things
we use: auto at 50 miles/hr = 70 kilowatts (a gallon of gas has
about 130 million joules); cooking range =12,000 watts; microwave=
1,400 watts; color TV= 350 watts).
• total annual human energy use -- about 4 x 1020
Joules or about 1014 kWhr. This is a good reference number
to keep in mind for thinking about the energy needs of human society
compared to the amount of energy conveniently available from various
sources.
Conclusion: So what is energy?
I’ve been discussing energy primarily in terms of examples
that hopefully make the concept very real and concrete, connecting
it closely to your own experience of the world. But energy itself,
abstracted out from specific instances to form a general principle,
is a very subtle notion, difficult to pin down as a particular kind
of “stuff”. What is energy, really? There’s something
very deep and mysterious about the fact that it is always conserved
through such a huge array of vastly different processes and even
though it takes widely different forms. Somehow nature must be “keeping
track” of something in order to make sure that the books always
balance to give the same amount of energy before and after each
process or transformation that occurs. We do something similar with
money or with tickets for carnival rides. Each ride requires a certain
number of tickets to make it possible, and the tickets give us a
tangible object that makes it easy to keep track and make sure that
an activity (ride) doesn’t occur unless the right number of
tickets are available to make it happen. The case with energy is
similar, only for energy we don’t really even know what the
tokens actually are! We just know that somehow the numbers always
balance when we do the calculations and add everything up. It’s
worth pondering the mystery of how nature keeps track so that the
numbers always balance. As a way to focus this mystery, we can think
of a specific process such as a photon being created (where it didn’t
exist before) when an electron in an atom drops down to a lower
energy level. The photon is created out of the energy stored in
the interaction between the electron and the nucleus of the atom,
and somehow nature keeps track and knows just how to make the right
frequency of photon based on the energy given up from the atom.
So the frame of mind I hope you take away from all this is to see
more things in your daily life in terms of the flow of energy into
and out of different parts of your world. Everything we ever do
involves a transformation of energy from one form to another. So
you can really think about where the energy follows a path from
one thing to another as you interact with stuff in your life: as
you take a bite of food, turn on an appliance, ride your bicycle
up a hill, or drive your car to work.
Recommended reading:
• Feynman, Leighton, Sands. The Feynman Lectures on
Physics, vol. 1. (Chapter 4 - Conservation of Energy).
• Hobson, Art. Physics: Concepts and Connections. New
Jersey, Prentice Hall, 1995. (Chapter 6).
• Physics Today, April, 2002. (Special issue on the
energy challenge).
• von Baeyer, Hans Christian. Warmth Disperses and Time
Passes: The History of Heat. New York: Random House, 1998. (Chapters
1-4).
|

